The importance of PERSEO’s research approach is essentially due to the fact that the results obtainable will be much more reliable, cheaper and faster than those obtainable from in vitro diagnoses and animal experiments. The PERSEO platfrm is made up of:

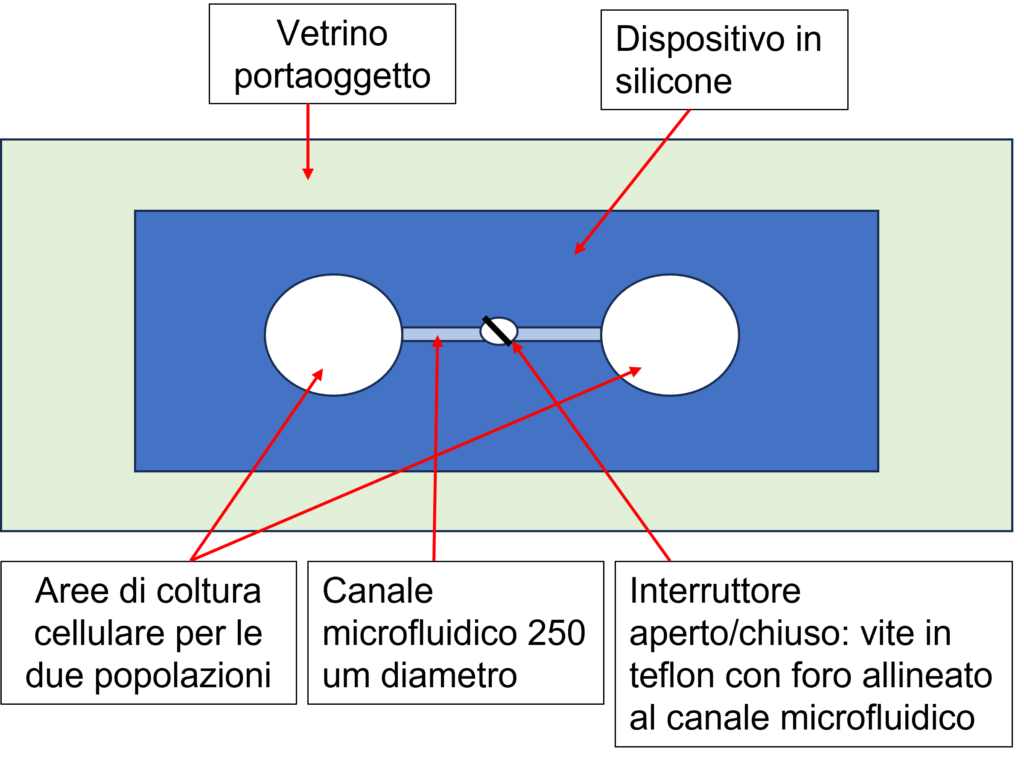
Two prototypes of the microfluidic device were built during the project. The first prototype of the microfluidic device (on the left) was made of silicone rubber (Dimethylpolysiloxane, PDMS), and with a thickness of about 10 mm. The device has been welded to a glass slide, to allow full compatibility with adapters for microscopy stages. The chip is formed by two areas on which the two populations of cell cultures can be plated. The two culture areas are connected by a constant section channel placed transversally about half the thickness of the PDMS block. Furthermore, the device provides for the presence of an open/closed switch formed with a Teflon screw. For the manufacture of the device, to ensure standardization in the preparation, a constructive scheme for the mold was prepared. Once the structure of the mold had been defined, we proceeded with its construction. The mold is then placed on a heated plate (for the polymerization of the PDMS) in correspondence with a glass Petri dish containing the components for the formation of the silicone rubber.
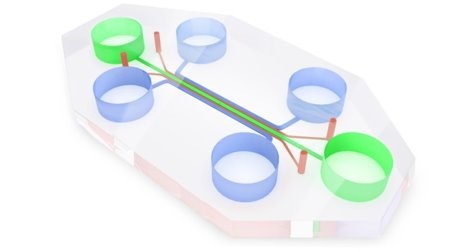
The second prototype of the microfluidic device was however produced in PDMS to allow the gaseous exchange necessary for cell cultures, and transparent to allow observation under a microscope. It includes 5 culture chambers, in fluidic contact with each other through a series of microchannels, and in contact with reservoirs for the culture medium. The cell populations were plated on both sides of the device (in blue), using instead the central channel (green) as a switch, being able to add medium and thus bring the two populations into contact at defined times. The devices were then welded onto 6-well optical bottom multiwell plates.
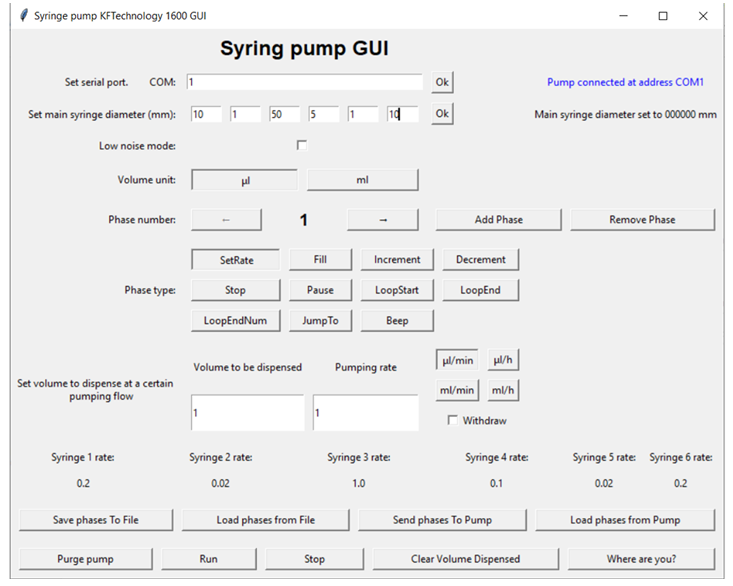
The Syringe Pumpe was positioned inside the incubator, consequently in order to allow the complete management of its functions, a Python language software with a graphical interface was created. In particular, the volume of fluid to be dispensed in a given observation interval must take into account both the volume of the chip chamber and the “dead” volume of the connection tubes. Based on this, the diameter of the chosen syringe is determined. The software defines the volumetric flow rate of the fluid dispensed which must not exceed the value of 1 µL/min in order not to deteriorate the cells as a result of shear stress.
Neuronal and pancreatic cells have been used as a biological model in the PERSEO project. The cells are placed in chemical communication with each other. Murine neuroblastoma cells (N1E-115) were used as a neuronal model, while murine pancreatic beta cells (MIN6) were used as a model of pancreatic functions in order to evaluate the chemical exchanges between the two cell populations related to the metabolism of glucose.

An experimental protocol for the model of the Neuron-pancreas axis has been developed. A case study of simulated metabolic damage inside the chip was evaluated and analyzed in the PERSEO project. Specifically, neuronal and pancreatic cells were placed in chemical communication. Murine neuroblastoma cells (N1E-115) were used as a neuronal model, while murine pancreatic beta cells (MIN6) were used as a model of pancreatic functions in order to evaluate the chemical exchanges between the two cell populations related to the metabolism of glucose. With the aim of recreating a typical condition of diabetes such as hyperglycemia, MIN6 murine insulinoma cells were treated in vitro with streptozotocin (STZ) for 24 hours. Metabolic damage was induced by STZ treatment, because this chemical compound is particularly toxic to beta cells and is used both to induce diabetes in animal models and as a model of induction of Alzheimer’s and memory impairment related to metabolic disorders of glucose. Initially, the potential cytotoxic effect on the STZ cell line was evaluated through the MTT cell viability assay. From a concentration of 3 mM, STZ causes a significant reduction in cell viability (p<0.005). In particular, the first toxic concentration of STZ capable of inducing damage to our cellular system was 4.6 mM. Accordingly, 5 mM concentration of STZ was used in the experiments. Subsequently, N1E115 murine neuroblastoma cells and MIN6 insulinoma cells were plated on the chip, a prototype provided by partner CNR-IFN.
In these experiments, the exchange between the equilibrium chambers was maintained, thanks to the use of the same volume of medium. After 24 hours, the microscopic acquisition process was started (1 frame every minute), in order to evaluate the morphology and motility of N1E115 cells. The MIN6 cells were treated with STZ as previously indicated and, after 24 hours, the conditioned medium of the same was brought into contact with the N1E115 cell line in which its medium had previously been removed. At this point, the acquisition of the frames was repeated, in order to evaluate whether the conditioned medium was able to induce morphological alterations of the N1E115 cells. The video shows images related to the different experimental conditions. As can be observed, treatment with STZ alters the cellular structure of MIN6; the conditioned medium of MIN6 cells treated with STZ induces modifications of the cellular structure of N1E115 neuroblastoma cells, suggesting that the molecules released following the insult are able to negatively impact the structure and functionality of these cells.

